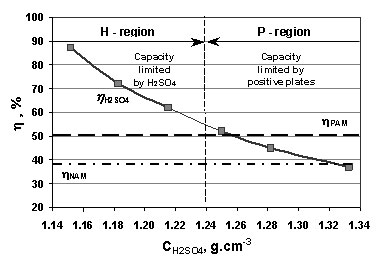
Broad temperature adaptability of vanadium redox flow battery-Part 3: The effects of total vanadium concentration and sulfuric acid concentration - ScienceDirect

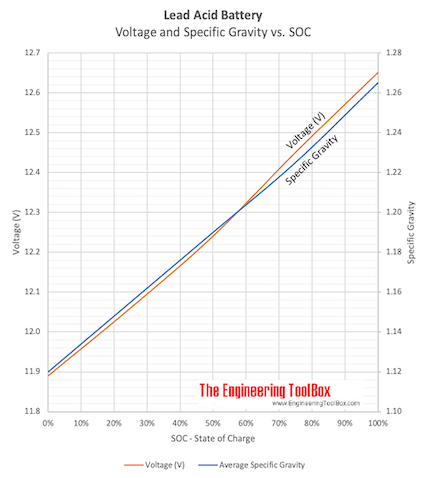
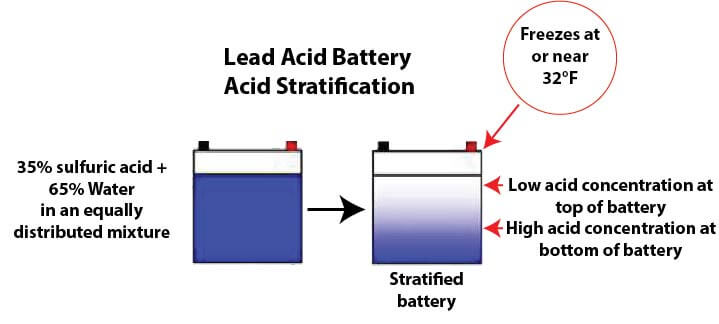
Measuring the density and specific gravity of battery acid in lead acid batteries :: Anton Paar Wiki
Recondition a dead car battery — Ricks Free Auto Repair Advice Ricks Free Auto Repair Advice | Automotive Repair Tips and How-To
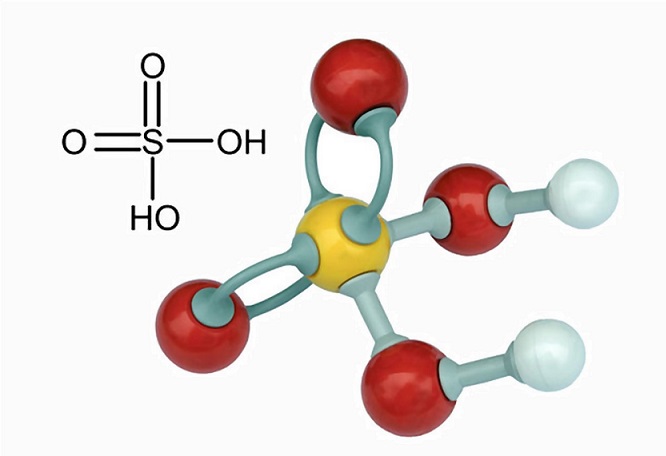
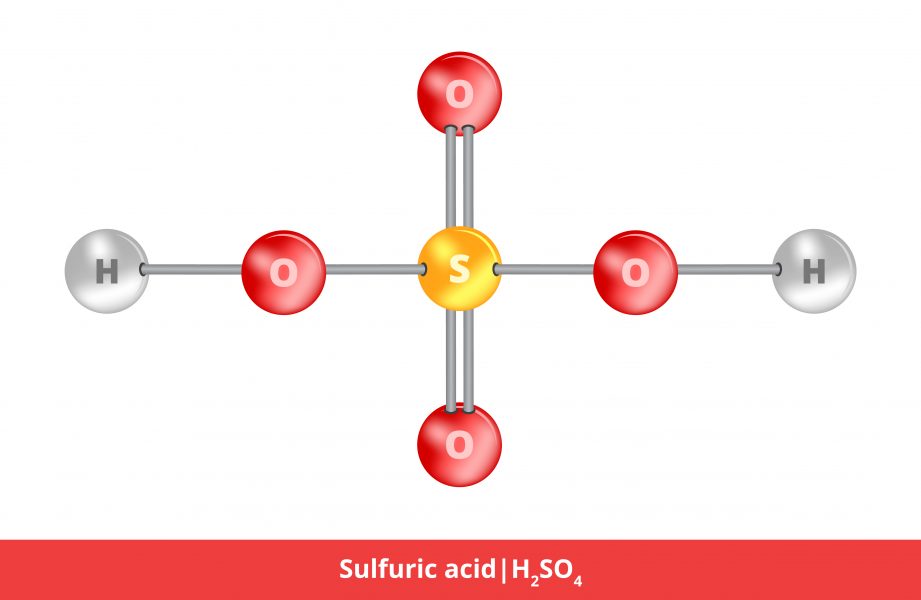
How to increase the Sulfuric acid concentration in battery acid which usually contains 35% sulfuric acid and 65% water - Quora

Selection of stainless steels for handling sulphuric acid (H2SO4) – British Stainless Steel Association
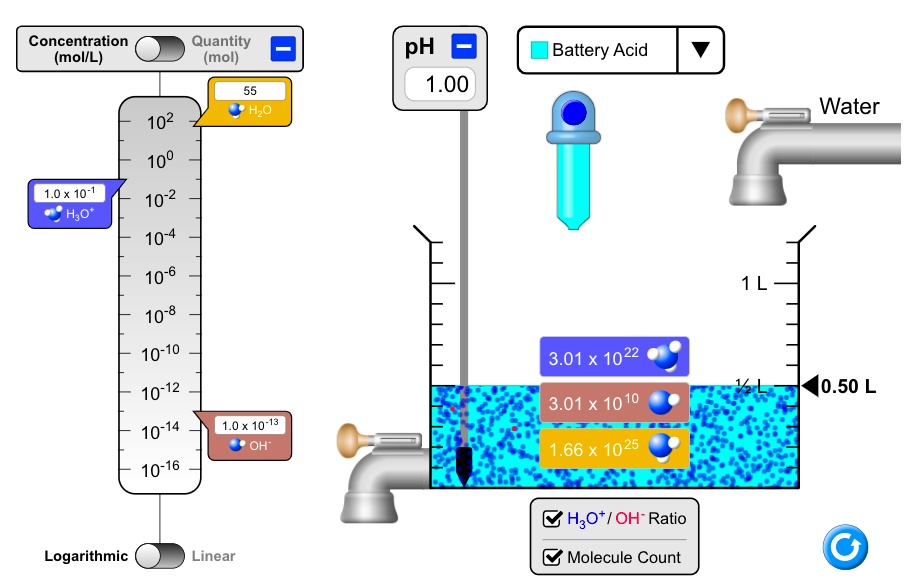
SOLVED: Concentration (mollL) Quantity (mol) pH 1.00 Battery Acid HLO Water 102 100 10-2 10-4 10-6 10-8 10-10 10-12 10-14 10-16 1.0 x 10-1 H;o" 3.01 x 1022 0.50 L 3.01 X 10 10 1.0 x 10-13 OH" 1.66 x 1025 H3ot/ OH-Ratio Logarithmic Linear Molecule Count

inorganic chemistry - What is the freezing point of sulfuric battery acid? - Chemistry Stack Exchange

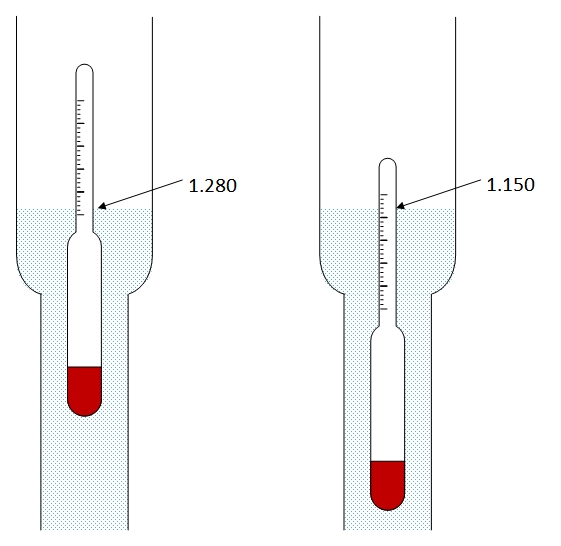
SOLVED: The lead-acid battery has the net reaction Pb PbOz + 4H+ + 2S042- = 2PbSOa 2HzO The concentration of sulfuric acid in the lead storage battery of an automobile over time


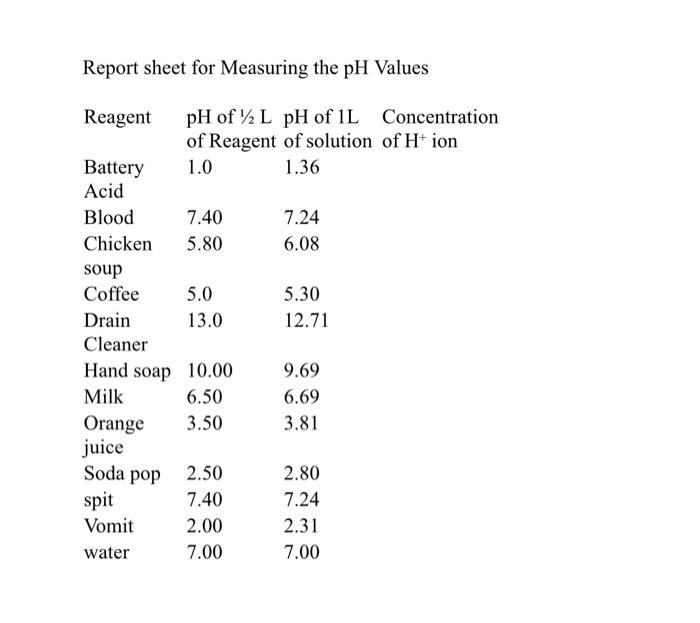

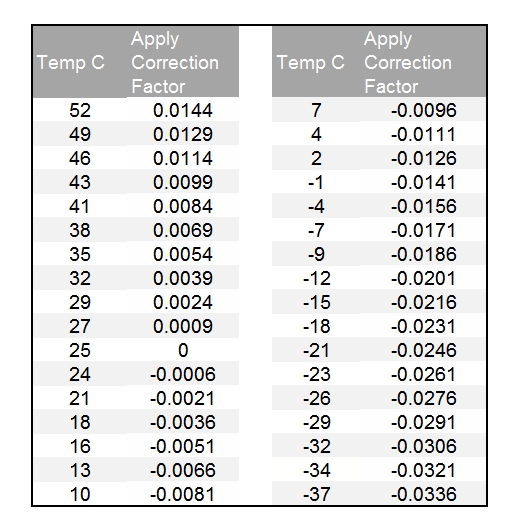

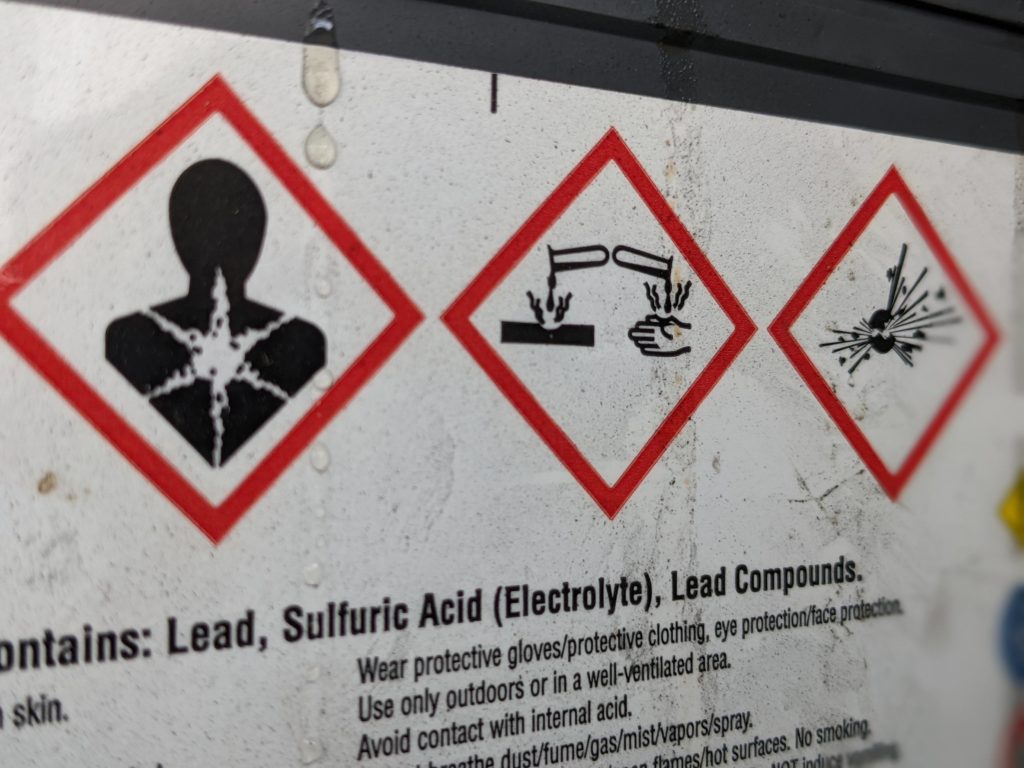

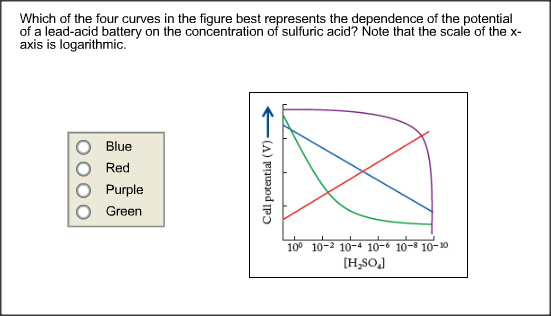
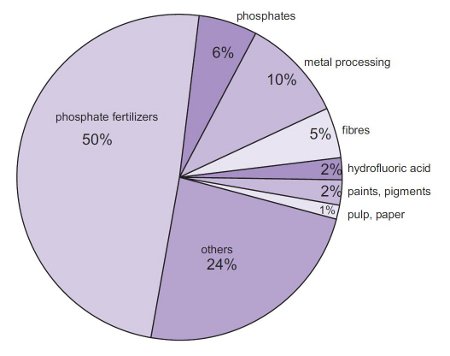
:max_bytes(150000):strip_icc()/car-battery-recycling-container-with-warning-notices-battery-acid-flusco-household-waste-recycling-centre-cumbria-uk-121814398-57a4e5055f9b58974a7355d8.jpg)